
Acticor Biotech, a clinical stage biotechnology company involved in the acute phase of thrombotic diseases, including acute ischemic stroke, today announced that it has raised €7 million in addition to its Series B which is now completed at €22,3 million. This additional funding will enable the company to expand ACTIMIS, a phase II trial in acute ischemic stroke with its lead candidate glenzocimab (ACT017) in the US.
The financing was led by a new investor Go Capital. Existing investors Newton BioCapital, CapDecisif Management and Anaxago also participated into this additional funding.
Simultaneously as closing its Series B financing, Acticor Biotech acquired 100% of AVCare’s shares. AVCare is a start-up diagnostic company, founded by Pr. Serge Timsit and Jean-Marc Herbert and financed by SATT Ouest Valorisation and Go Capital, which is developing a stroke blood biomarker. Acticor Biotech and SATT Ouest Valorisation enter into a sub-licensing agreement which gave worldwide rights to Acticor to develop and exploit the stroke biomarker.
Leila Nicolas, Investment Director of Go Capital will now join the Board of Directors of Acticor Biotech and she commented: “It was a very good opportunity for AVCare to be integrated into a promising biotech such as Acticor. We are also excited to participate into the financing of Acticor Biotech and look forward to getting the results of the phase II clinical development in acute ischemic stroke as well as expanding it into the US.”
Gilles Avenard, President of Acticor Biotech, added: “We are delighted to have raised additional equity from specialised healthcare investors who will enable us to expand our ongoing Phase II, ACTIMIS, in the US. Our pre-IND consultation with the FDA in the summer was encouraging enough to take the decision to file an IND in the coming months.”
For the closing of the series B financing, Agile Capital Markets acted as financial advisors for Acticor Biotech.
For more information, go to: https://acticor-biotech.com/
 In the presence of the President of the University Rennes 1, Mr. David Alis and the Vice President of the Brittany Region in charge of Energy Transition, Mr. André Crocq, KEMIWATT inaugurated its first organic RedOx battery beyond its walls.
In the presence of the President of the University Rennes 1, Mr. David Alis and the Vice President of the Brittany Region in charge of Energy Transition, Mr. André Crocq, KEMIWATT inaugurated its first organic RedOx battery beyond its walls.

David Alis President of Rennes 1, André Crocq Vice President of the Brittany Region in charge of the Energy Transition, Guillaume Chazalet President of KEMIWATT.
Within the SERU project, financed by the Brittany Region, KEMIWATT organic stationary energy storage system (8kW – 24kWh) was installed on the Beaulieu university campus. It is connected to photovoltaic panels and to a building allowing to test in real conditions of use, different storage applications such as load transfer, peak-shaving or backup.
KEMIWATT wishes with this battery RedOx organic demonstrate all the advantages of this technology developed from the research of the University of Chemistry Rennes 1:
• high simplicity and robustness of the solution with very low maintenance;
• a long life (20 years) and a very large number of cycles (> 20,000 cycles);
• high safety guarantees (no risk of fire, no release of gas, no risk of explosion), low sensitivity to high
temperatures;
• respect for the environment with its recyclable molecules, no rare earths or precious materials;
• the ability to dissociate power and energy;
Since its creation in 2014, KEMIWATT has been accompanied in its development by Demeter Ventures, GO CAPITAL, Pierre-Yves Divet and SATT Ouest Valorisation.
KEMIWATT, 11 allée de Beaulieu, CS 50837 – 35 708 Rennes Cedex
www.kemiwatt.com

The procedures were performed at Rouen University Hospital and Clinique Pasteur in Toulouse (France) and precede the first clinical study of its kind to be conducted in Europe
Rouen, September 24, 2019 – Robocath, a company that designs, develops and commercializes cardiovascular robotic systems for the treatment of vascular diseases, today announces the success of its first two coronary angioplasties performed with assistance from its R-One™ robotic platform. The procedures were performed in France at Rouen University Hospital by Professor Durand and Professor Sabatier and at Clinique Pasteur in Toulouse by Doctor Fajadet.
R-One™ assists interventional cardiologists in performing coronary angioplasties. The robotic platform is designed to facilitate and enhance interventional procedures performed for the patient and offers a better working environment for physicians and the entire medical team.
This prospective clinical study is the first of its kind to be conducted in Europe. It involves six European centers, three of which are based in France (Rouen University Hospital, Caen University Hospital, Clinique Pasteur in Toulouse). The study will involve 60 patients and aims to demonstrate the safety and efficacy of the robotic-assisted platform R-One™ in coronary angioplasties.
Dr Philippe Bencteux, president and founder of Robocath, says: “These two successes mark a new era in the way interventional cardiology is practiced in Europe today. It is a project we have worked on for several years and the entire team and I are extremely proud of the success of these two procedures.”
Professor Eric Durand, interventional cardiologist at Rouen University Hospital, comments: “I feel particularly privileged to have been given the opportunity several years ago to participate in this project and to now arrive at this first procedure in a human with excellent results.”
“I am delighted with the success of these procedures. It is rewarding to see Rouen University Hospital continuing to support cutting edge innovations in the medical sector, nearly twenty years after conducting our first aortic valve implantation in the same operating room,” said Alain Cribier, Professor Emeritus in Interventional Cardiology and Inventor of TAVI (Transcatheter Aortic Valve Implantation).
Dr Jean Fajadet, co-director of the Interventional Cardiology Unit at Clinique Pasteur in Toulouse, continues: “I am very happy with the success of these procedures and confident regarding the study results. We are on the cusp of a major revolution in the field of interventional cardiology, thanks to the possibilities opened up by vascular robotics that we are developing at Clinique Pasteur.”
Professor Rémi Sabatier, interventional cardiologist at Caen University Hospital, adds: “It was an honor for me to participate to this first robotized angioplasty. I’m delighted to share the joy of the teams who accompanied us on this adventure, which I’m looking forward to continuing at Caen University Hospital.”
Lucien Goffart, CEO of Robocath, concludes: “It is a French success story that has been supported by all of our employees and by highly invested medical and technical teams, to whom I would today like to extend my warmest thanks and congratulations. It is a crucial step in our development and one that will pave the way for a transformation of the sector. This transformation involves all of the players in the industry and inevitably leads us to think about this industry in broader terms, thanks to the new possibilities offered by robotics, particularly in terms of the interoperability of equipment.”
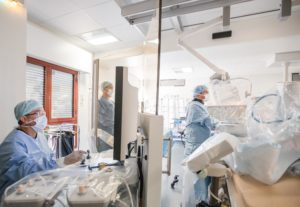
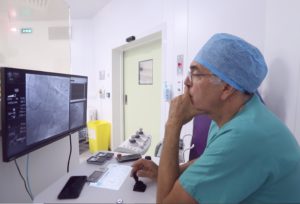
About Rouen University Hospital
The cardiology department of the University Hospital of Rouen is a reference for the management of
cardio-vascular diseases, the leading cause of death in the world and a major cause of call and consultation in the emergency department. The cardiology department was the first to implant a percutaneous aortic valve in 2002, a major medical advance. This intervention carried out by Professor Cribier and his team offers an alternative care to patients with severe aortic stenosis at high risk of surgical mortality. More than 250,000 people have benefited from this technique in the world to date. Rouen University Hospital intends to continue this dynamic of research and innovation internationally.
About Clinique Pasteur, Toulouse
As the first independent private clinic in France, Clinique Pasteur has been making use of its expertise in cardiology for more than 30 years, with its team of professionals striving for excellence and innovation every day. The clinic has a cardiac center created by health care professionals with patients in mind and was designed to optimize patient care in cardiology. Ultra-modern interventional cardiology units are equipped with highly technical facilities and, starting this year, now integrate robotic-assisted angioplasty in their daily practice.
About Robocath
Founded in 2009 by Philippe Bencteux, MD, Robocath designs, develops and commercializes robotic solutions to treat cardiovascular diseases. As an active player in the evolving medical robotic industry, these innovative solutions aim to make medical procedures safer thanks to reliable technologies, while complementing manual interventions.
R-One™ is the first solution developed by Robocath. It uses a unique technology that optimizes the safety of robotic-assisted coronary angioplasty. This medical procedure consists of revascularizing the cardiac muscle by inserting one or more implants (stents) into the arteries that supply it with blood. Every 30 seconds, somewhere in the world, this type of procedure is performed.
R-One™ is designed to operate with precision and perform specific movements, creating better interventional conditions. Thanks to its open architecture, R-One™ is compatible with market-leading devices and cath labs. It received the CE marking in February 2019.
Robocath aims to become the world leader in vascular robotics and develop the remote treatment of vascular emergencies, guaranteeing the best care pathway for all.
Based in Rouen (France), Robocath has more than 25 employees and is financially supported by regional investment funds (GO CAPITAL, NCI, Normandy participations), national investment funds (M Capital, Supernova Invest), by several business angels and financial institutions (Caisse d’épargne, BNP Paribas, Crédit Agricole) and Bpifrance.
PRESS CONTACTS
| Morgane Le Mellay |
| morgane.mellay@robocath.com |
| +33 (0)6 34 40 91 25 |
| Juliette Schmitt dos Santos / Celine Gonzalez |
| juliette@ala.com / celine@ala.com |
| UK: + 44 1273 675 100 / US: + 1 617 202 4491 |

Rouen, September 3, 2019. – Robocath, a company that designs, develops and commercializes cardiovascular robotic systems for the treatment of vascular diseases, announces today that it has raised €5 million ($5.5M) to support the rollout of its robotic platform in strategic European target markets. The company has also hired Lucien Goffart as CEO.
With twenty years of experience in interventional cardiology, Lucien Goffart has held a number of key positions in leading companies in the sector. He also has a strong track record of supporting international marketing of new products. His résumé notably includes a position as Business unit manager for France at Boston Scientific, a post he occupied for the last four years, and his recent appointment as an executive board member at Electroducer, a French manufacturer of vascular devices.
Philippe Bencteux, founder & president of Robocath, said, “I’m delighted to welcome Lucien Goffart to the company at such a key moment for us with the commercial launch of our first robotic platform. His experience in the industry is undeniable. This appointment has been made at a particularly auspicious time, with our only direct competitor, Corindus Vascular Robotics, having been bought out by Siemens for $1.1 billion (€1bn). I would like to thank our longstanding shareholders, who have once again demonstrated their belief in us by raising this new capital. We are also grateful for the support shown by new shareholders.”
Lucien Goffart, CEO of Robocath, added, “It’s an honor for me to begin work today as CEO of Robocath. I’m impressed by the progress the company has made since it was founded and I’m excited to be working with such a young and dynamic team. R-One™ is a unique product that offers several advantages to physicians and, by extension, to patients. It has significant potential on the market, and I’m looking forward to starting clinical studies and writing a brand new page in the history of interventional cardiology.”
The €5 million ($5.5M) capital injection was provided by longstanding shareholders (GO CAPITAL, NCI, Normandie Participations, M Capital and Supernova Invest) and new funds (Caisse d’Epargne Normandie Innovation, Crédit Agricole innove en Normandie and Unexo, a subsidiary of the Crédit Agricole group). The company previously raised €6.4 million ($7M) in 2017. This new injection will allow Robocath to lay the groundwork for rolling out the R-One™ robotic platform in a number of target European countries.
About Robocath
Founded in 2009 by Philippe Bencteux, MD, Robocath designs, develops and commercializes robotic solutions to treat cardiovascular diseases. As an active player in the evolving medical robotic industry, these innovative solutions aim to make medical procedures safer, thanks to reliable technologies, while complementing manual interventions.
R-One™ is the first solution developed by Robocath. It uses a unique technology that optimizes the safety of robotic-assisted coronary angioplasty. This medical procedure consists of revascularizing the cardiac muscle by inserting one or more implants (stents) into the arteries that supply it with blood. Every 30 seconds, somewhere in the world, this type of procedure is performed.
R-One™ is designed to operate with precision and perform specific movements creating better interventional conditions. Thanks to its open architecture, R-One™ is compatible with market leading devices and cath labs. It received the CE marking in February 2019.
Robocath aims to become the world leader in vascular robotics and develop the remote treatment of vascular emergencies, guaranteeing the best care pathway for all.
Based in Rouen (France), Robocath has more than 25 employees and is financially supported by regional investment funds (GO CAPITAL, NCI, Normandy participations), national investment funds (M Capital, Supernova Invest), by several business angels and financial institutions (Caisse d’épargne Normandie,
BNP Paribas, Crédit Agricole) and Bpifrance.
SINAY has been developing for 3 years a big data platform, including artificial intelligence as its core engine, dedicated to maritime industry players. SINAY has just closed Seed round with European and American investors to accelerate the development of professional applications for maritime activities in Europe and North America.
“SINAY bridges the gap between maritime expertise and data technologies. Our platform accelerates the way to create maritime data centric applications for Ports, Shipping, Oil & Gas, Marine Renewable Energy and Fisheries” said Yanis SOUAMI – CEO SINAY.
SINAY Platform is an end-to-end platform for companies to build, deploy and scale maritime data applications to accelerate the ocean economy digital transition. Customers can access a powerful data infrastructure including High Performance Computing Cluster, data sets, algorithms, dashboards and standardize applications.
They have a choice among 6.000 data sets (including real-time AIS vessel positions, oceanographic and wildlife data, as well as 35.000 buoys, amongst others) and 60 dedicated maritime algorithms (including pollution modelling, marine traffic analyses, risk assessments, and wildlife habitat modelling …).
Thanks to SINAY Platform, they experience quick ROI on their maritime data by activating standardize applications from our marketplace or by building their own applications. There are many use cases such as real-time environment monitoring during offshore construction, risk assessment for subsea cables, port logistic optimization or vessel fuel consumption reduction.
SINAY closed a 1,6 M€ Seed round table thanks to Alpana Ventures (Swiss/USA), Mer Invest of Crédit Maritime (France), Plug & Play Ventures (USA) and with its historical investors, Normandie Participations from the Normandy Region (France), Crédit Agricole via CA’in – Crédit Agricole Innove en Normandie (France) – and BNP (France). This new fund-raising round table will allow SINAY to push the development of its platform and to accelerate its internationalization, especially in United States with its two investors based in the Silicon Valley.
“Alpana Ventures sees a huge opportunity in providing all kind of Big Data / Predictive Analytics to the Maritime Industry. SINAY and its team have been gathering Data for years and their expertise is second to none. We are looking forward working with them.” said Alexander FRIES – Alpana-Ventures.
![]()
Press Contact : Claire SIMON – claire.simon@SINAY.fr – +33 (0)2.50.01.15.50 www.sinaymaritimedata.com
Rouen, France, May 21, 2019 – Robocath, a company that designs, develops and commercializes cardiovascular robotic systems for the treatment of vascular diseases, announces today that Pr Haude, Dr Lorgat and Pr Nef have joined its medical advisory board. All are global experts and recognized leaders in interventional cardiology.
The board plays a key role in defining the clinical strategy of the company. Their clinical work will contribute to the worldwide growth of robotics in interventional cardiology.
Philippe Bencteux, president and founder of Robocath, said: “I’m particularly proud and pleased to welcome Pr. Michael Haude, Dr. Faizel Lorgat and Pr. Holger Nef to our medical advisory board (MAB). As experts they have demonstrated their strong commitment and enthusiasm to being part of this new page in history. We all share the same vision regarding the future of interventional cardiology and the key role that our robotic platform will play in this field.”
The new appointees are:
 Pr. Michael Haude – Städtische Kliniken Neuss – Neuss, Germany
Pr. Michael Haude – Städtische Kliniken Neuss – Neuss, Germany
Head of the cardiac catheterization laboratory at the Neuss Clinic for the 25 last years, past chairman of the Working Group on Interventional Cardiology (AGIK) at the German Society of Cardiology, PCR and ESC board member and EAPCI president from 2016 to 2018. Currently an EAPCI board member, Pr. Haude has published more than 300 scientific articles.
“I’m very proud to be involved in the very first steps in robotics in Europe and to initiate clinical research in Germany in this important new innovation in interventional cardiology.”
 Dr. Faizel Lorgat – Netcare Christiaan Barnard Memorial Hospital – Cape Town, South Africa
Dr. Faizel Lorgat – Netcare Christiaan Barnard Memorial Hospital – Cape Town, South Africa
Interventional cardiologist at Christiaan Barnard Memorial Hospital since 1998. One of the first users of a robotic system for electrophysiology, Dr. Lorgat has performed more than 1,600 procedures with this robotic assistance over a period of 6 years and is an active research contributor in this field.
“It’s an honour to join the medical advisory board of Robocath together with other world-renowned interventional cardiology experts. I’m convinced that robotics is definitely the future for our field. I’m looking forward to initiating clinical research and to sharing my experience worldwide.”
 Pr. Holger Nef – Giessen University Hospital – Giessen, Germany
Pr. Holger Nef – Giessen University Hospital – Giessen, Germany
Head of the cardiac catheterization laboratory at Giessen University Hospital for the last 10 years. Director of the department for structural heart disease at the Herz-Kreislauf-Zentrum, Rotenburg, Germany. President of the Working Group on Interventional Cardiology (AGIK) at the German Society of Cardiology since 2015, Pr. Nef is the author of numerous scientific publications and plays an active role in professional education.
“I am looking forward to using robotic technology in a clinical setting and I am honoured to be part of this extraordinary group of colleagues.”
These three experts join Robocath’s existing MAB members: Dr. Alberto Cremonesi (Humanitas Gavazzeni Hospital – Bergamo BG, Italy), Pr. Alain Cribier (Emeritus Professor and TAVI founder, Rouen University Hospital – Rouen, France), Dr. Jean Fajadet (Pasteur Clinic – Toulouse, France), Pr. Eric Durand (Rouen University Hospital – Rouen, France), Pr. Rémi Sabatier (Caen University Hospital – Caen, France), Pr. Gregg Stone (Columbia University Hospital – New-York, United States).
About Robocath
Founded in 2009 by Philippe Bencteux, MD, Robocath designs, develops and commercializes robotic solutions to treat cardiovascular diseases. As an active player in the evolving medical robotic industry, these innovative solutions aim to make medical procedures safer, thanks to reliable technologies, while complementing manual interventions.
R-One™ is the first solution developed by Robocath. It uses a unique technology designed to optimize the safety of robotic-assisted coronary angioplasty. This medical procedure consists of revascularizing the cardiac muscle by inserting one or more implants (stents) into the arteries that supply it with blood. Every 30 seconds, somewhere in the world, this type of procedure is performed.
R-One™ is designed to operate with precision and perform specific movements creating better interventional conditions. Thanks to its open architecture, R-One™ is compatible with market leading devices and cath labs. It received the CE marking in February 2019.
Robocath aims to become the world leader in vascular robotics and develop the remote treatment of vascular emergencies, guaranteeing the best care pathway for all.
Based in Rouen (France), Robocath has more than 25 employees.
www.robocath.com
• IFB-088 observed to have a favorable safety, tolerability and pharmacokinetic profile in the Phase 1 trial
• IFB-088 is advancing into a Multiple Ascending Dose part of the phase 1 trial to support a future Phase 2 trial in Charcot-Marie-Tooth disease
InFlectis BioScience SAS, a drug discovery company committed to the development of innovative therapeutics harnessing the Integrated Stress Response for the treatment of a broad range of diseases, today announced favorable results from the Single Ascending Dose (SAD) part of the Phase 1 trial (P188) that evaluated six single doses of IFB-088 administered by oral route. IFB-088, a selective inhibitor of stressed-induced PPP1R15A/PP1c phosphatase complex, is planned to be developed primarily for the treatment of Charcot-Marie-Tooth disease.
The SAD part of the double-blind, randomized, placebo-controlled Phase 1 trial evaluated the safety, tolerability and pharmacokinetics of six doses of IFB-088 in a total of 48 healthy volunteers. Results demonstrated IFB-088 to be well tolerated at doses ranging from 2.5 mg to 60 mg daily, with linear human pharmacokinetics over the dose range tested. IFB-088 was observed to show a favorable safety and tolerability profile with no serious adverse events at all doses tested within the trial.
Based on the favorable results of the SAD part, the Company is preparing to initiate the Multiple Ascending Dose (MAD) part of the Phase 1 study. The MAD will be conducted according to a double-blind, randomized, placebo-controlled, multi oral ascending dose design and will assess the safety, tolerability and pharmacokinetics of three doses of IFB-088 administered to healthy volunteers over 14 days.
Following the successful completion of the Phase 1 study planned by year end, InFlectis BioScience will transition the IFB-088 program into Phase 2 clinical trials to test the drug treatment in patients with Charcot-Marie-Tooth diseases 1A and 1B. Based on preclinical evidences and proofs of concept in CMT1A and CMT1B animal models, the European Commission and the Food and Drug Administration (FDA) have both already granted orphan drug designation (ODD) to IFB-088 for the treatment of Charcot-Marie-Tooth diseases. Additional preclinical data will be presented at Peripheral Nerve Society annual meeting in Genoa, Italy in June 2019 and will highlight the activity of IFB-088 as a potent and selective agent to improve the clinical signs of neuropathy in CMT1A and in CMT1B.
During the remainder of 2019, preparation for the phase 2 program of IFB-088 in Charcot-Marie-Tooth patients will also include a pre-Investigational New Drug meeting with the U.S. Food and Drug Administration and a Protocol Assistance with the European Medicines Agency to discuss the IFB-088 development strategy in CMT.
Anne Visbecq, Chief Medical Officer of InFlectis BioScience SAS said: “We are pleased with the outcome of the IFB-088 SAD part of Phase 1 trial, which confirms the good tolerance profile of IFB-088. We are planning to initiate the MAD part in healthy volunteers during the summer of 2019 with completion expected in the fourth quarter. This trial will be important in the development plan of IFB-088 in Charcot-Marie-Tooth disease”.
Notes for editors:
ABOUT IFB-088 (also known as Sephin1)
IFB-088 is a first-in-class orally available small molecule drug candidate with a validated mechanism of action and a promising pharmacokinetic profile for targeting the central and peripheral nervous system. IFB-088 improves protein homeostasis following a stress (e.g. misfolded protein accumulation, oxidative stress…) activating the Unfolded Protein Response or the Integrated Stress Response observed in several neurodegenerative disorders, including CMT.
IFB-088 is targeting the stress-induced PPP1R15A/PP1c phosphatase complex involved in dephosphorylation of translation initiation factor eIF2α. Thus, IFB-088 regulates the protein translation rate in stressed cells to a level manageable by the available cellular proteins that assist in protein folding (so-called “chaperones”), thereby restoring proteostasis. IFB-088 is strikingly specific for stressed cells, avoiding persistent inhibition of protein synthesis in normal, non-stressed cells.
ABOUT THE IFB-088 PHASE 1 P188 CLINICAL TRIAL
The Phase 1 P188 clinical trial, which is being conducted in France, is a randomized, double-blind, placebo-controlled, single and multiple ascending dose study of IFB-088, designed to evaluate the safety, tolerability and pharmacokinetics of IFB-088 in healthy subjects. The Phase 1 P188 clinical trial will enrolled 72 healthy adult volunteers into 6 SAD cohorts of 8 subjects with IFB-088 given orally as single doses ranging from 2.5 mg to 60 mg daily, and 3 MAD cohorts of 8 subjects with IFB-088 given orally for 14 days.
ABOUT INFLECTIS BIOSCIENCE (www.inflectisbioscience.com)
InFlectis BioScience is a France-based clinical stage company committed to the development of innovative therapeutics harnessing the Integrated Stress Response for the treatment of a broad range of diseases. Dysregulation of Integrated Stress Response (ISR) signaling has important pathologic consequences linked to neurodegenerative diseases, inflammation and cancers. Harnessing the ISR by the pharmacological modulation of eIF2α dephosphorylation to restore the cellular homeostasis, is a new frontier pioneered by InFlectis BioScience since 2013, based on many years of academic development. Through its unique and selective mode of action on the ISR, InFlectis BioScience is developing a breakthrough pharmacological approach that, for the very first time, has the potential to treat a wide range of diseases whose etiology lies in different genetic mutations. Thus, the benefit of modulating eIF2α dephosphorylation with small chemical compounds, like IFB-088, was demonstrated in validated animal models of Charcot-Marie-Tooth (CMT), Amyotrophic Lateral Sclerosis (ALS) and Multiple Sclerosis (MS). The primary focus of InFlectis BioScience is the development of novel therapeutics that improve the life of patients with CMT, the most common inherited neurological disorder. InFlectis is also contemplating other promising therapeutic indications among neuro-degenerative diseases, inflammation and cancers. Based in Nantes in Western France, InFlectis BioScience is part of the science park of the economic area of Nantes Atlantique.
INFLECTIS BIOSCIENCE SAS
Philippe Guédat
President and CEO
philippeguedat@inflectisbioscience.com
Pierre Miniou
Chief Operating Officer
pierreminiou@inflectisbioscience.com
qsdqsd